Diffusion dialysis - waste alkali recovery technology
Product Details:
Diffusion dialysis - waste alkali recovery technology Price And Quantity
- 1000.0 USD ($)
- 1
Diffusion dialysis - waste alkali recovery technology Trade Information
- Telegraphic Transfer (T/T) Letter of Credit (L/C)
- No
- Africa Asia Australia Central America North America South America Eastern Europe Western Europe Middle East
- All India
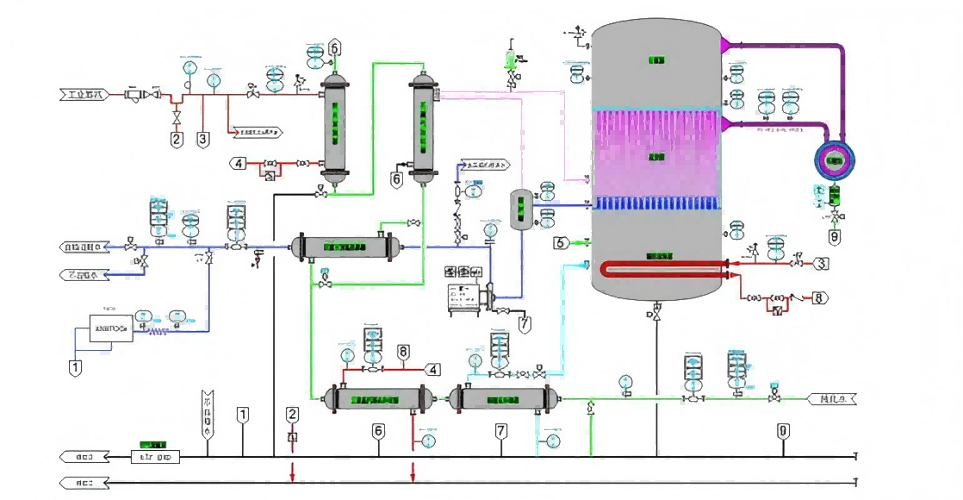
- The whole device is a series of structural units composed of a certain number of membranes. Each unit has a cationic homogeneous membrane separated into a dialysis chamber and a diffusion chamber. Countercurrent operation is adopted. When the waste lye and the receiving liquid (tap water) are passed into both sides of the cationic homogeneous membrane, the concentration of alkali and its salt on the waste lye side is much higher than that on the water side. However, the membrane has selective permeability to cations, so under the effect of the concentration difference, the cations on the waste alkali side are attracted and enter the water side smoothly through the membrane pore channel. At the same time, according to the requirements of electric neutrality, anions are also included, because the hydration radius of OH- is relatively small, and the charge is less. Other anions have a larger hydration radius and more charge, so OH- will preferratively pass through the membrane, so that the alkali in the waste liquid will be separated.
Product Description
Diffusion dialysis recovery of waste alkali and recovery of waste acid are the application of dialysis principle, are driven by concentration difference, the difference is that used as diffusion dialysis membrane is not anion exchange membrane but cation exchange membrane. As shown in the figure below, when both sides of the cationic homogeneous membrane pass into the waste lye and the receiving solution (tap water), the concentration of alkali and its salt on the waste lye side is much higher than that on the water side. Therefore, due to the existence of concentration gradient, the waste alkali and its salt have a tendency to penetrate the diffusion chamber. However, the membrane is selective and will not allow each ion to pass through with equal opportunities. First of all, the cation exchange membrane skeleton itself is negatively charged, which has the characteristics of attracting positively charged hydrated ions and repelling negatively charged hydrated ions in solution. Therefore, under the effect of the concentration difference, the cation on the waste alkali side is attracted and smoothly enters the water side through the membrane pore channel. At the same time, according to the requirements of electric neutrality, negatively charged ions will also be included, because the hydration radius of OH- is relatively small, the charge is less; Other anions have larger radii, so OH- will preferentially pass through the membrane, so that the alkali in the waste solution will be separated.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+












 Send Inquiry
Send Inquiry