Pharmaceutical use and water integration system
Product Details:
Pharmaceutical use and water integration system Price And Quantity
- 1 Unit
- 10000.0 USD ($)/Unit
Pharmaceutical use and water integration system Trade Information
- Telegraphic Transfer (T/T) Letter of Credit (L/C)
- 1 Unit Per Week
- 3 Months
- No
- Asia Australia Central America North America South America Eastern Europe Western Europe Middle East Africa
- All India
- FDA/cGMA
Product Description
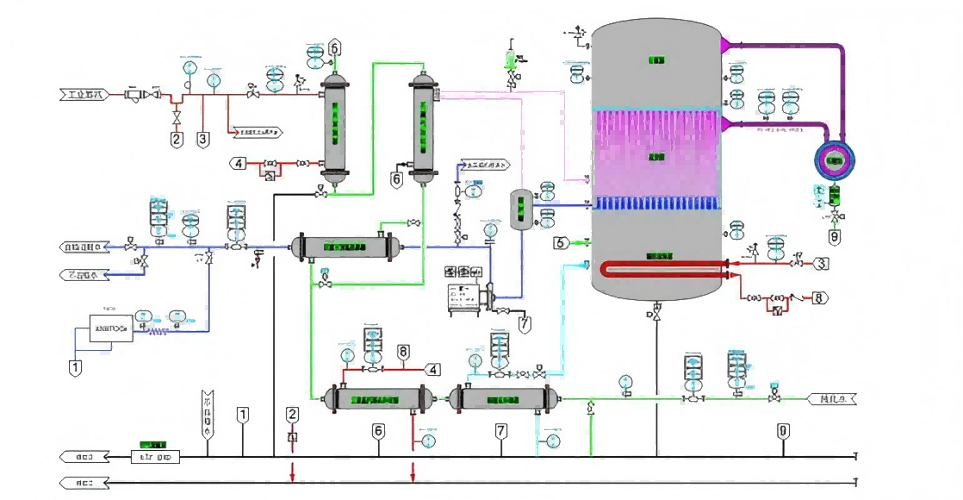
1.Pre-processing unit
The modular pretreatment unit is designed according to the inlet characteristics. The robust design is designed to pretreatment the water according to the final treatment requirements. Provide stable performance and ensure the maximum availability of the system.
2.Pure water and water for injection
Pure water (PW) or water for injection (WFI) is produced for all pharmacopoeia, generating PW or WFI using the core technology of reverse osmosis and continuous electric DI. Water for injection is generated by a secondary membrane system with increased ultrafiltration function and does not go through a distillation process.
3.Store and distribute the modules
Standard distribution functions can be implemented in a single module and deliver the quality and quantity of the customer. The distribution system solution integrates all the technical convergence, instrument control in one system, and maximizes the need for space. The overall system design provides reliable operation, continuous monitoring, while maintaining optimal energy efficiency and reducing operating costs without compromising quality.
4.Pure steam generator
The steam generator can provide the dry saturated steam conforming to the Pharmacopoeia specifications, the vertical design of the pure steam generator has the DTS evaporator, and the separator ensures that no microdroplets or impurities pass through the product steam. The generator is designed to have an energy recovery and a non-condensing gas evaporation system to provide a constant high-purity steam.
5.Vertical hot-pressed distilled water machine
The vertical hot press was designed and manufactured in full accordance with cGMP guidelines, FDA and EMEA requirements for the production of injection water (sterile water without heat source). The key thrust of this system is the efficiency of energy conservation to ensuring the maximization of energy utilization without affecting quality.
6.Integrate the automation systems
The whole process can be monitored and controlled to control the output quality parameters and grasp the performance information.
7.Strict material technology
316L / 304 stainless steel meeting the material and process requirements, ASME BPE or ISO20037.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+








 Send Inquiry
Send Inquiry Call Me Free
Call Me Free